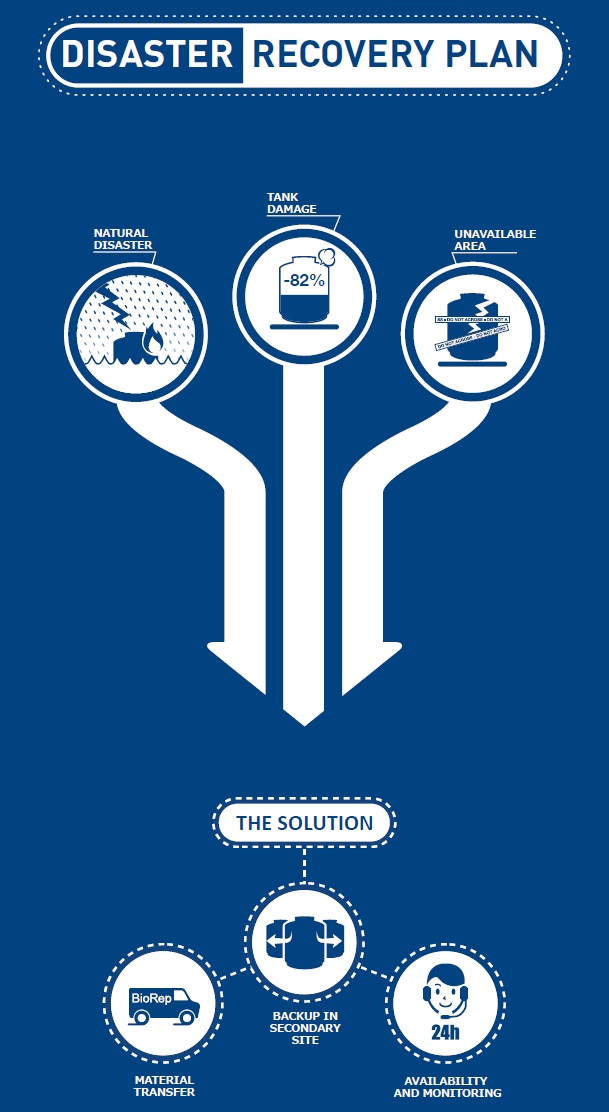
Disaster recovery plan
Legislative Decree no. 191 of 6 November 2007 implementing Directive 200423 EC on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells, provides for the intervention of a third party body capable of making up for the temporary unfitness of laboratories and/or storage area in the event of unexpected catastrophic events or permanent and serious damage to one or more tanks of nitrogen or interruption of the supply of liquid nitrogen for various reasons (Art. 24).
BioRep was the first private facility in Italy authorized in 2012 by the Ministry of Health to carry out, as a third party, activities of transport, storage and preservation of hematopoietic stem cells and reproductive tissues, as a support to the emergency plan (DISASTER RECOVERY PLAN), in favour of Tissue Institutes (current Ministerial authorization: No. 0036180-23122016-DGPRE-MDS-P).
BioRep has all the necessary requirements to carry out this type of activity:
- adequate cryogenic room, cryogenic back-up containers,
- dedicated monitoring and control service,
- specific standard procedures
- specialized personnel available 24-365 days a year.
The handling and transport of the tanks is carried out in compliance with the regulations for the transport of biological material, according to UNI EN ISO 9001:2015 standards, using drivers in possession of a Professional Training Certificate and vehicles equipped according to ADR regulations.
The emergency management plan is customized according to the needs of the individual structures, in accordance with the Biobank managers. The transfer of samples in the appropriate cryobiological containers respects the highest quality standards in all phases and critical points of the intervention.